Medicinal plants are well-known for their bioactive compounds, with various parts possessing distinct medicinal properties. One such medicinal plant is Curcuma caesia Roxb., commonly known as Black turmeric, and is popular among ethnic groups in Nepal and India because of its diverse medicinal values. Researchers are exploring this hidden talent and have identified components such as camphor, 1, 8 Cineole, ar-curcumene, β-elemene, borneol, bornyl acetate, and α-terpineol as major phytochemicals. The rhizomes and leaves of this plant have demonstrated antibacterial, antioxidant, anticancer, and anti-inflammatory properties attributed to their bioactive phytochemicals. C. caesia, with its unique properties, can emerge as a potential alternative to various synthetic drugs used in wound treatment, cancer therapy, bacterial infections, and immune system enhancement. This study aims to shed light on the recognition of this valuable plant, which is still hidden in the soils of Nepal and India. This article compiles various research findings on the medicinal properties of the phytochemicals found in C. caesia. It explores the potential applications of this plant using advanced technologies leading to economic growth within the field of medicine.
Article Open access20 November 2025
Article 13 August 2022

Article 18 December 2024
Discover the latest articles, books and news in related subjects, suggested using machine learning.
About 80% of the global population relies on traditional herbal medicine as a source of healthcare due to its easy availability, low cost, high efficiency, non-narcotic nature, and minimal side effects [1]. Plants serve as the main source of such traditional remedies, which have been used for centuries. The bioactive components of the different parts of such plants contribute to their medicinal value, a factor that consistently captures the attention of researchers [2]. In recent years, there has been growing interest regarding the plant-based bioactive compounds that can be the potential treatment of various complications like bacterial infection [3], cancer [4], inflammation [5], asthma [6], endothelial dysfunction [7], hyperglycemia [8] and ulcers [9].
One such plant genus with noteworthy medicinal bioactive phytochemicals is Curcuma commonly known as turmeric. Turmeric is a popular traditional medicinal herb used as a spice, especially in South Asian cuisines like Nepali and Indian which has proven pharmacologic effects like anti-inflammatory, antibacterial, and antioxidant [10]. Among various species of this ethnomedicinal species, Curcuma Caesia or C. caesia has potent medicinal properties and has been used in the traditional system of medicine such as Ayurveda and Vaidhya traditions [11]. It is a perineal herbaceous plant that grows wild in the forest of the Hills and Terai region of Nepal, but it is also cultivated by the native people for its medicinal value [12]. This endangered herb is found in other Southeast Asian countries, and China [13].
The bioactive phytochemicals and potential therapeutic benefits of C. caesia are being extensively researched across multiple countries worldwide. Different in vivo and in vitro studies have explored the different bioactivities of the phytochemicals found in this herb. However, despite its local acclaim, the medicinal potential of C. caesia remains unexplored scientifically, leaving numerous promising therapeutic benefits untapped and unverified [14].
Various species of Curcuma are utilized in the production of diverse formulations such as tablets, emulsions, and powders, aimed at treating a spectrum of ailments including inflammatory bowel disease, arthritis, ulcers, H. pylori infection, psoriasis, and beyond [15, 16]. These formulations have been in use as supplements to bolster immunity against a multitude of diseases [17]. Nonetheless, comparable formulations derived from C. caesia remain insufficiently explored. This plant harbors a wealth of beneficial components that could serve as promising alternatives to synthetic drugs in addressing a wide array of complications. This review aims to summarize the recent studies on bioactive phytochemicals and their therapeutic activities along with the potential application of C. caesia in medicine.
Curcuma caesia is classified within the Plantae kingdom, specifically under the subkingdom Viridaeplantae. It belongs to the Phylum Tracheophyta Sinnott and is categorized further into the Subphylum Euphyllophytina. At the class level, it falls under Magnoliopsida (monocotyledons) (commelinids). Taxonomically, it is placed within the order Zingiberales, the family Zingiberaceae, the subfamily Zingiberoideae, the tribe Hedychieae, and the genus Curcuma, with the specific species identified as Curcuma caesia Roxb.[18, 19]. This species is recognized by various vernacular names [20]. In Nepali, it is referred to as ‘Kaalo Beshar’ or ‘Kaalo Haledo,’ while in Sanskrit, it is known as Rajani, Nishaa, Nishi, and Raatri. In India and Bangladesh, it goes by the name ‘Kala Haldi’, while in English, it is commonly referred to as Black Turmeric. The plant ranges from 0.5 to 1.0 m in height, with an underground ovoid tuberous rhizome and an erect aerial shoot bearing leaves and flowers. The rhizome, measuring 2 to 6 cm in diameter, is sessile, flattened laterally, marked with warts and root scars, and features circular arrangements of scaly leaf remnants, resembling growth rings as shown in Fig. 1.

Microscopic analysis of the circular transverse section of the rhizome has revealed that it consists of distinct layers: a thick-celled epidermis covered by a detached cuticle, a broad parenchymatous cortex, an underdeveloped endodermis, and a starch-filled parenchymatous pith. Fibro-vascular bundles traverse through these regions, with collateral vascular bundles associated with thin-walled fibers. Fibrous adventitious roots are present, while leaves are oblong-lanceolate, featuring a ferruginous purple middle region and ivory-colored petioles. The inflorescence is a dense spike preceding leaf opening, with green bracts and deep pink coma bracts that age to crimson. Flowers are smaller, pale yellow-pinkish, with a 10 to 15mm long calyx and a long tubular corolla displaying a pale yellow, semi-elliptic, three-lobed lip. The plant can grow to a height of 0.5 m to 1.0 m. It is differentiated into an underground large ovoid tuberous rhizome often called rootstock and an erect aerial shoot with leaves and flowers.
The Curcuma genus, including Curcuma caesia, has been utilized for centuries for both nutritional and therapeutic purposes. Initially valued by indigenous groups, it has now garnered attention among modern generations. Its rhizome, highly esteemed in Ayurveda for medicinal properties, contains a potent medicinal essence [21]. While leaves and stalks are consumed in salads, soups, and teas for their benefits, dried rhizomes are used mostly in powdered form for flavor and nutrition enhancement in foods. Boiling rhizomes creates a nutritional tonic that is used to build up the immune system [22]. Meanwhile, C. caesia has been proven to be a rich source of mineral elements, like Iron, Potassium, Sodium, Calcium, Magnesium, and Phosphorus. Along with the mineral elements, the crude extract of the rhizomes is found to have a high amount of protein, fibers, fats, and carbohydrates [23, 24].
Figure 2 summarizes the traditional application of the whole plant.
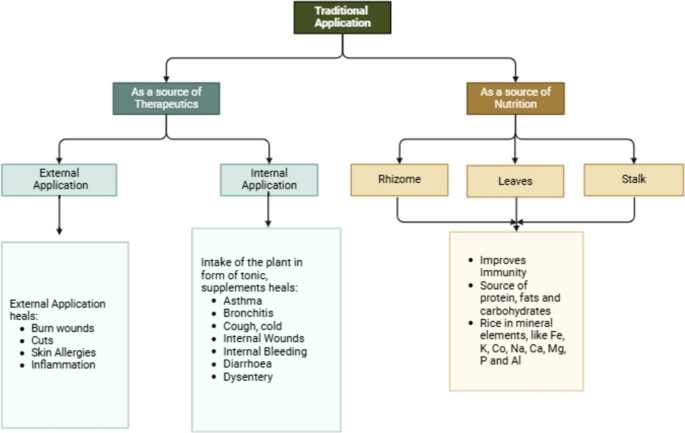
In Nepal, the rhizomes, leaves, and stalks are used by people as a source of medicine to treat various skin-related problems like allergies and inflammations. They are also applied to burn and cut wounds as they possess antibacterial properties that are believed to prevent any kind of bacterial infection in the wound. Additionally, people use the plant as a supplement in the form of a tonic or directly consume the rhizomes to cure different diseases such as asthma, cough, cold, bronchitis, lung issues, diarrhoea, and dysentery. Moreover, these rhizomes are also used to treat internal wounds and are believed to aid blood clotting post-surgery. Women post-childbirth consume them to heal surgical wounds [20, 24, 25].
The species within the Curcuma genus are known to be abundant in bioactive phytochemicals like alkaloids, flavonoids, and terpenoids. Apart from this, C. caesia is enriched with bio components such as amino acids carbohydrates, phenols, steroids, glycosides, saponins, and tannins [26]. The rhizome of the C. caesia plant is extensively utilized for extracting bioactive components. Similar to other species of the genus Curcuma, the oleoresin extract of the rhizome of C. caesia contains two main active components: volatile oil and curcuminoids [27, 28]. The volatile oil is claimed to comprise 30 components with camphor, ar-turmerone, (Z)-β-ocimene, ar-curcumin, 1, 8-cineole, β-element, borneol, bornyl acetate, and γ-curcumene as the major constituents [28]. The three primary curcuminoids are demethoxycurcumin, curcumin, and bisdemethoxycurcumin [10].
Meanwhile, Vairappan et al. investigated the presence of secondary metabolites in the rhizomes of C. caesia for the first time in 2012 [29]. They were successful in isolating nine sesquiterpenes from the rhizomes which are listed in Table 1. However, the same study claimed that diarylheptanoids, the major secondary metabolite of this genus were not present in C. caesia. A study reported that gas chromatography (GC) of C. caesia leaves revealed an oil yield of 0.8% and identified fourteen constituents, with major components including 1,8 Cineole (27–0%), Camphor (16.8%), Borneol (8.7%), α-Terpineol (5.2%) and β-Pinene (6.3%). Minor constituents included α-pinene, myrcene, limonene, linalool, β-elemene, β-caryophyllene, and (E)-methyl isoeugenol [30].Table 1 List of important bioactive compounds present in C. caesia
Full size tableTable 2 Biological activity of major phytochemicals present in C. caesia rhizome
A study comparing the ethanolic extracts of six Curcuma species found that C. caesia exhibited the lowest total curcumin content [31]. Additionally, high-performance thin-layer chromatography (HPTLC) fingerprinting highlighted the presence of camphor in the methanol extract of C. caesia, distinguishing it from C. longa and C. amada. However, curcumin, a marker for C. longa and C. amada, was absent in the methanol extract of C. caesia [32].
Along with this, research reported that methanol extract of the rhizome of C. caesia, contains 2- methylbenzene-1, 3-diol which was revealed by column and thin layer chromatography separation techniques [33]. The FTIR analysis of solvent extracts of this plant revealed the existence of functional groups such as O–H, C = C, C = O, C-O, C-H, N–H, and CH3 [26]. Meanwhile, Laser-Induced Breakdown Spectroscopy (LIBS) analysis conducted on C. caesia rhizome identified essential macronutrients such as Nitrogen, Sodium, Magnesium, and Carbon including the essential micronutrient, Chlorine. Additionally, it indicated the presence of non-essential micronutrients Silicon and Barium, typically absent in plant-derived products [34].
Furthermore, a study done by Pandey et. al discovered that a purified fraction from the crude extract of C. caesia having a molecular weight of 368 and a molecular formula C21H20O6 showed similar chemical and structural characteristics to that of curcumin (1, 7- bis (4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene3, 5-dione) [35]. The purified fraction was found to exhibit the highest antibacterial activity as compared to other biocomponents of the extract. Additionally, The GC–MS analysis showed that the major components present in the C. caesia were α-Santalol (46.90%), Retinal (10.72%), Megastigma-3,7(E),9-triene, Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl(4.38%), 5,8,11,14,17-Eicosapentaenoic acid, methyl ester, (all-Z)- (4.26%) tricyclo [8.6.0.0(2, 9)] hexadeca-3, 15-diene, trans-2, 9-anti-9, 10- trans-1, 10(3.26%) and various other compounds were identified as low level [36, 37]. Figure 3 shows the structure of major phytochemicals present in C. caesia.
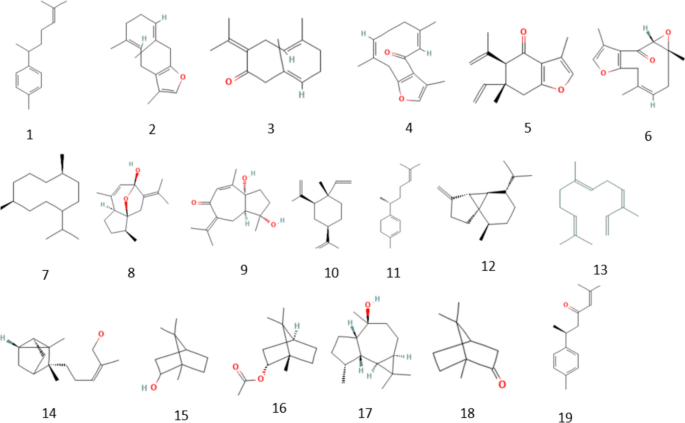
The secondary metabolites of herbal plants exert a definite physiological action on the human body and thus are widely used in human therapy for various diseases. Different types of major phytochemicals present and their specific biological properties have been listed in Tables 1, 2.
The therapeutic effect of medicinal plants is mainly attributed to their antioxidant properties that are linked to various phytochemical compounds as listed above. These compounds may exhibit different activities based on structural factors such as the quantity of phenolic and flavonoid groups, methoxyl or hydroxyl groups, and other features that interfere with oxidation processes by interacting with free radicals, binding catalytic metals, and acting as scavengers for reactive species. The antioxidant activities of C. caesia plant extract are mainly due to its total phenolic and flavonoid content. A qualitative comparison of different extracts revealed that the methanolic extract had the highest levels of total phenolic and flavonoid content [47].
The antioxidant activities can be determined by measuring DPPH free radicle scavenging activities, ABTS (2, 2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) free radical scavenging activities, ferric reducing ability, superoxide, and MTT (3-(4, 5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium bromide) antioxidant assay [48].
According to a report by Liu et al. [24], the methanolic extract of C. caesia rhizome exhibited significant natural antioxidant properties. The extract, at a concentration of 100 μg/mL, displayed comparable oxidative effects to Ascorbic acid and tert-butylhydroquinone at 25 μg/mL in the MTT Assay. In a comparative analysis of C. caesia extracts, the methanol extract showed the highest percentage of free radical scavenging activity via the DPPH assay, followed by the ethyl acetate extract. The methanol extract also demonstrated a high amount of metal ion chelating activity[43].
An in vitro evaluation of the antioxidant activity of C. caesia indicated that the methanol extract had a moderate IC 50 value compared to standard butylated hydroxytoluene [43].
The antioxidant property of methanol extract of the rhizome of C. caesia has been identified through in vivo studies as well. Another study has demonstrated the extract’s efficacy in scavenging free radicals in streptozotocin-induced diabetes in Wistar rats [49]. Treatment with the methanol extract of the rhizome inhibited lipid peroxidation in the diabetic rates, as indicated by the reduction in Thiobarbituric Acid Reactive Substances (TBARS) levels toward normal. This inhibition of lipid peroxidation is vital in preventing tissue injury and failure of endogenous antioxidant defense mechanisms. Moreover, the treated rats showed gradual healing in the beta cells of the pancreas after the treatment [49].
Furthermore, in a comparative analysis, C. caesia showed a higher phenolic content than C. amada. Its oxygen-reducing power, superoxide, ABTS, and DPPH radical scavenging activities were also superior to C. amada. [50].
The hexane and methanolic extract of C. caesia exhibited moderate Lipid Peroxidation (LPO) inhibitory activity, and the MTT assay showed activity profiles similar to the activity of vitamin C and Tertiary butylhydroquinone (TBHQ) [24].
A study has also found that the solar-dried sample of C. caesia has better antioxidant activity compared to the open sun-drying [51].
The antioxidant activity of phenols and flavonoids is attributed to several mechanisms, including their ability to donate hydrogen or electrons, chelate redox-active metal ions, modulate gene expression, and interact with cell signaling pathways [52]. However, research suggests that the predominant mechanism underlying their free radical scavenging capability is hydrogen donation by the extracts [53]. This ability stems from the chemical structure of phenolic compounds, characterized by a hydroxyl group attached to the benzene ring [54].
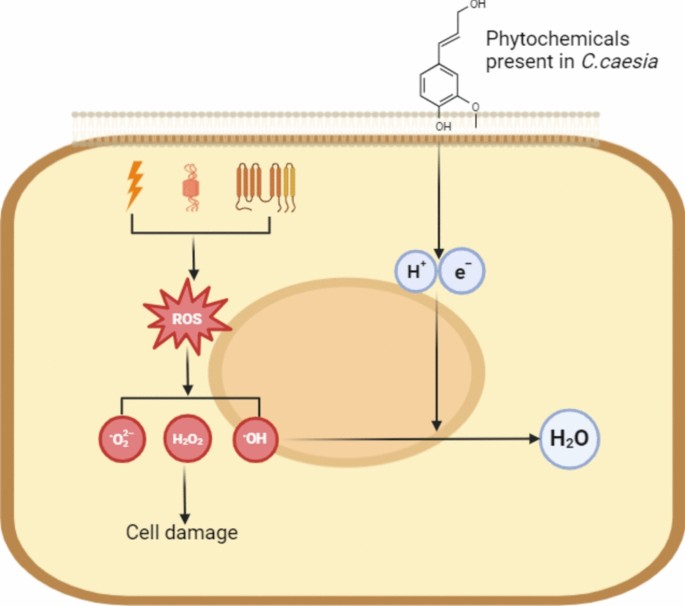
In the presence of the reactive oxygen species (ROS) in the cell, the bond between oxygen and hydrogen of the phytochemicals is disrupted, liberating hydrogen ions that become available to neutralize the nucleophilic radicals, thereby quenching the free radical activity as shown in Fig. 4 [55]. ROS typically arise from oxidative stress induced by various exogenous and endogenous factors, including radiation, microbes, and NADPH, which emerge as free radicals such as hydrogen peroxide, superoxide, or hydroxyl within the cell. These free radicals are known to damage cellular components, ultimately resulting in cell death. Conversely, when phytochemicals release hydrogen ions and free electrons, the generated ROS are converted into water or oxygen, thus preventing cell death [53].
Inflammation is a biological reaction of the immune system triggered by various factors leading to chronic disease or tissue damage.
In an in vivo experiment, the methanol extract of C. caesia demonstrated significant anti-inflammatory activity. It reduced the paw edema volume in Wistar rats with carrageenan-induced paw edema during the late phase (3 to 5 h), which is controlled by prostaglandins and leukotrienes. In a sub-acute model using cotton pellet-induced granuloma in Wistar rats, the extract also decreased the dry weight of the granuloma [56].
Proteins isolated from aqueous Soxhlet extraction of rhizome of C. caesia showed significant antioxidant activity which was found to be heat stable. When tested on the carrageenan rat paw model system it showed high anti-inflammatory activity at a dose level of 100mg/kg [57].
The mechanism behind the anti-inflammatory action of C. caesia extract lies in the inhibitory effect of its phytochemicals on the production of lipid mediators such as prostaglandin and thromboxane [58]. These mediators play an important role in triggering inflammation and pain during normal physiological functions. This inhibition is achieved either by preventing the release of Arachidonic acid or by inhibiting the production of cyclooxygenase (COX) enzyme in the prostaglandin production pathway as shown in Fig. 5 [59]. Arachidonic Acid is the fatty acid present in the human cell membranes that is released during membrane injuries and is oxygenated by COX enzymes, leading to the production of prostaglandin and thromboxane [60].
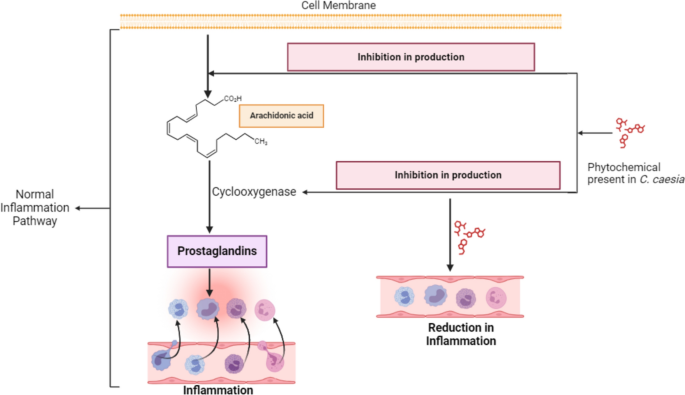
Phytochemicals such as flavonoids, alkaloids, and phenols inhibit leukocyte accumulation and neutrophil degranulation, directly reducing the release of arachidonic acid and histamine. Additionally, quercetin, a derivative compound of flavonoid has been shown to inhibit the production of COX and lipoxygenase (LOX). This compound also suppresses the production of Nuclear Factor kappa B (NFκB), and Nitric Oxide (NO) which are known to intensify the inflammations [57].
Thrombolysis is a complex process of breaking down clots within the body, involving interactions between clot components and the surrounding plasma. A thrombolytic agent is capable of dissolving the clots by triggering the activation of plasminogen in the blood plasma, thereby facilitating smooth blood flow [61]. An in vitro analysis of the ethanolic extract of C. caesia indicated significant clot dissolution action in comparison to the negative control, Streptokinase and C. amada. The observed thrombolytic activity might stem from the activation of plasminogen. Nevertheless, the precise mechanism and the specific active components through which the extract operates remain uncertain [62].
The antibacterial properties of the medicinal herb are due to the presence of various flavonoids and polyphenolic compounds. These compounds enable the formation of a membrane-attacking complex (MAC) with the bacterial cell walls, thereby impeding microbial growth. Both the inhibition of bacterial growth and antioxidant capabilities of different C. caesia extracts rely on the dosage administered. An experiment performed to study the antibacterial activity of six different rhizome extracts of C. caesia, revealed that in Gram-positive bacteria, the acetone extract of C. caesia demonstrated the highest effectiveness against S. aureus. The antibacterial activity of the acetone extract against S. aureus and S. haemolyticus was comparable to that of standard antibiotic streptomycin. Among the gram-negative bacteria, the chloroform extracts of C. caesia exhibited optimal activity against S. marcescense. The antibacterial activity is more prominent in gram-negative bacteria [26]. According to a study done by Juariah et al., it was found that the ethanol extract of C. caesia had the highest inhibitory action against S. aureus [63]. This study was conducted to reduce the nosocomial infection caused due to the hospital environment, therefore the antibacterial test was done against different gram-positive and gram-negative bacteria [63]. The phytochemicals like saponins, terpenoids, and tannins were found to be responsible for this inhibitory action of the C. caesia ethanol extract.
Furthermore, research reported that the essential oil from the rhizome of C. caesia was most effective against the multidrug-resistance (MDR) strain A. baumannii with MIC value of 0.09 to 6.25 µg/mL [64]. The oil also showed significant inhibitory activity against the other two MDR strains which were E.coli and K. pneumoniae. Juariah et al. analyzed the mechanism of antibacterial activity of C. caesia in their recent study [65]. The activity was checked against Klebsiella pneumoniae, the pathogen responsible for causing pneumonia. According to this study, there was the deposition of nucleic acids and proteins outside the cell of the pathogen indicating the damage in the cell wall. The cellular components were leaked out either due to the rupture in the cell wall or due to the changes in the permeability of the cell membrane causing the bacteria to die [66, 67].
The change in size and shape of the cell was also observed due to the exudation of the cellular components caused by the damage to the cell wall. A healthy cell wall of bacteria protects the cytoplasmic membrane keeping the cell in its form and maintaining the osmotic pressure preventing the cell from death [68, 69].
Figure 6 shows the schematic illustration to represent the proposed antibacterial mechanisms of C. caesia rhizome extract. When the extract interacts with bacteria, the extract initiates damage to the bacterial cell wall through its active phytochemicals. Consequently, the permeability of the cell wall increases, facilitating the diffusion of hypo-osmotic surrounding fluid into the cell, resulting in cell swelling [70]. This swelling elevates the osmotic pressure within the cell, ultimately leading to cell death as cellular components are expelled from the cell [67].
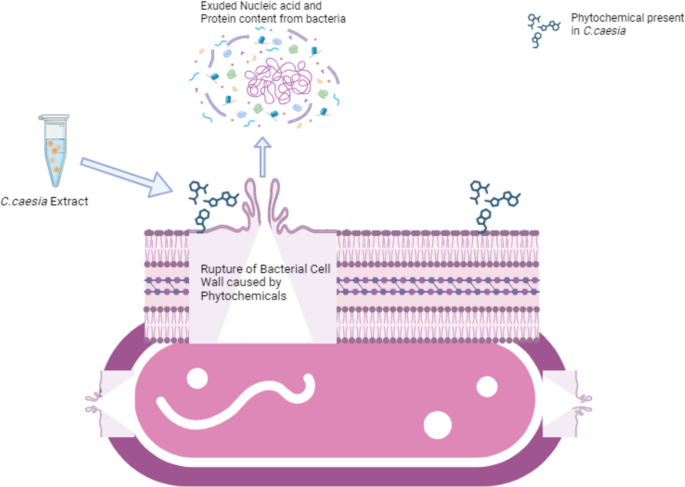
Also, the absorbance of the extract of C. caesia rhizomes by the pathogen cell was found to be directly related to the extract’s concentration. This suggests that phytochemicals like flavonoids, saponins, terpenoids, etc. are responsible for the change in the cell wall [71]. Meanwhile, the absorbance at 280nm is higher than the absorbance at 260nm in the same study signifying that the protein loss from the cell exceeded that of nucleic acid.
Curcumin inhibits the transcription of Epstein-Barr virus key activator BamH fragment Z left frame 1 (BZLF1) protein in Raji DR-LUC cells. It serves as an inhibitor of inosine monophosphate dehydrogenase (IMPDH), demonstrating potent antiviral properties. Curcumin effectively suppresses HIV-1 LTR-directed gene expression without significantly affecting cell viability. It also significantly reduces the acetylation of the HIV Tat protein by p300, thereby hindering HIV-1 replication [72].
According to the study on molecular docking of potential COVID-19 main protease inhibitors, performed using Auto dock 4.2, with Lamarckian Genetic Algorithm, some of the compounds such as demethoxycurcumin, oleuropein, and curcumin which are the main bioactive components of C. caesia, have the best potential to act as COVID-19 main protease inhibitors. The binding energies obtained from the docking of 6LU7 with demethoxycurcumin, oleuropein, and curcumin were −7.99, −7.31, and −7.05 kcal/mol respectively [73].
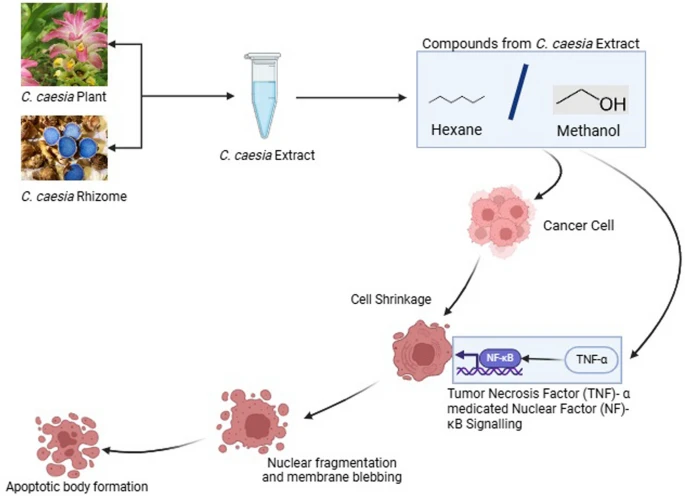
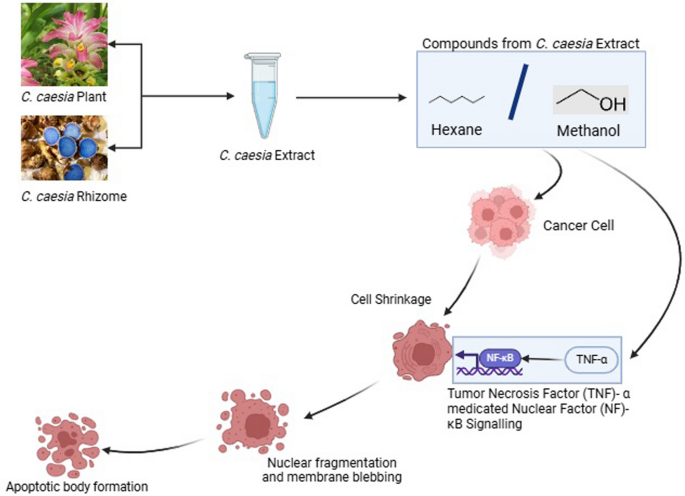
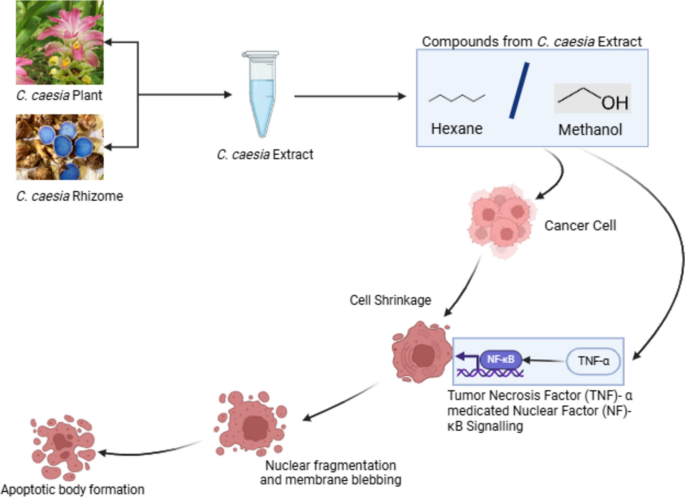
Cancer is the unwanted proliferation of cells which has become one of the leading causes of death globally. The medications for cancer are still under study. Research indicates that C. caesia also has phytochemicals that can show action against cancer. The in vivo study carried out by Sharan and group found out that the methanol extract of C. caesia has components such as phenolic, flavonoids, terpenoids, tannins, etc. which show anticancer properties through the Tumor Necrosis Factor (TNF)-α medicated Nuclear factor(NF) –κB signaling [74]. This study was carried out in Diethylnitrosamine (DEN) induced BALB/c mice and the anticarcinogenic effects of the extract were determined by analyzing the TNF-α levels in serum and NF- Κb binding activity in nuclear extracts of the liver.
Additionally, an in vitro test suggested that the hexane extract of the rhizome of C. caesia possesses significant antioxidant and anticancer properties [75]. Through MTT Assay on HepG2 cell line and with the help of western blot and flow cytometry this study proved that the hexane extract can induce HepG2 cell line arrest along with cell apoptosis.
Similarly, another in vitro cytotoxicity assay of methanol extract of C. caesia rhizomes on Ehrlich’s ascites carcinoma (EAC) cell line showed direct cytotoxicity on the cell line [76]. The same study also did the in vivo test on EAC-treated mice which showed that the methanol extract of the rhizomes exhibited a significant decrease in tumour volume and weight in the EAC-treated mice. The study also found that treatment with methanol extract of the rhizome can increase the lifespan by 88.09% in the EAC-treated mice.
The assay targeting tumor cell proliferation demonstrated that the chloroform and ethyl acetate extracts of C. caesia significantly suppressed the growth of human tumor cells HCT-116 due to the presence of phenolic acids and flavonoid compounds [77].
Additionally, recent research works have focused on the nanotechnological applications of Curcuma caesia [78]. In this study, gold nanoparticles were synthesized from the ethanolic extract of the rhizome. The MTT assay revealed that both the crude extract and the synthesized gold nanoparticles exhibit significant cytotoxicity against breast cancer cell lines, specifically MCF-7 (ER +) and MDA-MB-231 (Triple Negative Breast Cancer, TNBC). [78]
Curcuminoids possess well-documented anti-inflammatory, antioxidant, and anti-cancer effects, inhibiting tumor growth, inducing apoptosis, and modulating cancer-related signaling pathways[76, 79]. C. caesia extracts exhibit cytotoxic effects on cancer cells, inducing cell cycle arrest, inhibiting proliferation, and promoting apoptosis, potentially through modulation of cellular signalling pathways [75]. The anti-cancer mechanism of the plant extracts has been demonstrated in Fig. 7. While these findings suggest the potential of C. caesia extracts for anti-cancer therapy, further research is needed to elucidate their mechanisms of action and therapeutic efficacy in clinical settings, emphasizing their potential as complementary treatments alongside conventional cancer therapies [77].
Infections from parasitic worms such as flukes, tapeworms, and roundworms are prevalent in both human beings and livestock, and they can cause serious health issues such as lymphatic filariasis, onchocerciasis & schistosomiasis, even leading to death [80, 81]. Therefore, drugs with anti-helminthic activity, which demonstrate efficacy against such parasitic worm infections, are used to treat them [81]. Preferably, an anthelmintic drug should cure the infection with a single dose and be free from any toxicity that could cause additional harm to the host. These features can often be found in natural resources that possess potential efficacy against such infections [82].
In a comparative assessment of C. amada and C. caesia anthelminthic activity, the ethanol extract demonstrated the most significant efficacy in inducing earthworm paralysis in a dose-dependent manner [83]. Piperazine citrate was taken as a standard drug for evaluation. Additionally, ethanol, petroleum ether, and dichloromethane extracts from both C. amada and C. caesia proved highly effective in causing earthworm mortality. During phytochemical analysis, the ethanol extracts of C. caesia tested negative for phenols and tannins, while the dichloromethane extracts tested negative for phenols exclusively.
Furthermore, in an experiment performed in an earthworm, the rhizome’s ethanol and chloroform extracts from C. caesia exhibited superior vermifuge activity compared to vermicidal effects, following a dose-dependent pattern in contrast to standard Albendazole. The phytochemical investigation of the ethanol extract revealed the presence of carbohydrates, glycosides, saponins, phytosterols, resins, phenols, and flavonoids. Meanwhile, the chloroform extract also indicated the presence of tannins [83].
Turmeric has been traditionally used for disorders associated with the digestive system. The bioactive compounds present in the plant are responsible for this medicating feature. A study reported that the ethanol extract of C. caesia has the potential to reduce gastric acid volume, pepsin, and total acidity. The study was performed on ulcer-induced albino rats pre-treated with Aspirin. The albino rats when treated with ethanol extract showed a significant increase in the production of gastric mucus [25, 84].
Agents with analgesic properties serve as pain relievers and impact both the central and peripheral nervous systems without inducing unconsciousness. An in vivo experiment conducted on Swiss albino mice, utilizing the acetic acid-induced writhing model (representing peripheral action) and the hot plate test (representing central action), showed that methanol extract of C. caesia functions as both a peripheral as well as central analgesic agent [56].
C. caesia has a history in folk medicine for addressing inflammation and asthma. A preliminary mechanistic study examining the smooth muscle relaxant impact of the plant’s ethanolic extract on rabbits and guinea pigs sheds light on its traditional use in asthma and vascular disorders. The relaxant action of the C. caesia extract may stem from its ability to hinder calcium release from intracellular stores and calcium efflux from the extracellular space. This implies that the extract’s relaxant effect operates independently of the epithelium [85].
Additionally, treatment using methanolic C. caesia extract notably extended the latent period of convulsions induced by histamine in guinea pigs, supporting the plant’s anti-asthmatic properties [86].
Curcuma caesia is investigated not only for the bioactive compounds, including curcumin, volatile oils, flavonoids, and alkaloids, known for their therapeutic benefits but also subjected to toxicological evaluation [87]. While moderate doses generally appear safe, higher concentrations and prolonged use raise some toxicological concerns. Acute toxicity studies, which aim to determine the lethal dose (LD50) after a single high exposure, examine the effects on key organs such as the liver, kidney, and heart. Results indicate that moderate doses of C. caesia do not cause significant toxicity; however, higher doses can lead to symptoms such as lethargy, appetite loss, and elevated liver enzymes, indicating liver stress[88]. While chronic toxicity studies assess the effects of prolonged exposure (28–90 days), high doses have been associated with liver toxicity due to elevated liver enzyme levels, potential DNA damage, and gastric irritation due to mucosal damage in the gastrointestinal (GI) tract [88]. While exact lethal doses have not been established, studies suggest that high doses may lead to organ damage, underscoring the need for controlled dosing to avoid adverse effects. Table 3 summarizes the pharmacological activities exhibited by different parts of the plant, highlighting various biological effects and the recommended lethal dose to achieve therapeutic benefits without causing harm.Table 3 Summary of pharmacological activities of different parts of C. caesia, outlining biological effects and method of isolation
The promising biomedical potential of C. caesia highlights valuable avenues for future research and development. Future studies could focus on the isolation and characterization of specific bioactive compounds, advancing the synthesis of pharmaceutical agents or nutraceuticals with targeted applications. Additionally, investigating the potential synergistic effects of C. caesia with existing therapies may offer insights into integrative medicine [91]. Advancements in nanotechnology and biotechnology hold particular promise for enhancing the medicinal efficacy of C. caesia in novel ways, as outlined in the following paragraphs.
Previous research has unequivocally demonstrated the different therapeutic properties in the rhizomes of C. caesia. These inherent qualities, crucial for effective wound treatment, present an exciting avenue for further exploration [92]. Harnessing these properties through C. caesia nanoparticles could amplify its therapeutic effects when integrated into wound-healing nanomaterials such as scaffolds, hydrogels, or grafts (xenografts, autografts, or allografts), potentially enhancing product efficacy overall [93, 94]. Comprehensive research on C. caesia in nanoparticle form could yield promising applications, including its use as an efficient drug delivery system for the targeted treatment of internal injuries or other health complications.
The biosynthesis of metallic nanoparticles using C. caesia represents an emerging area of interest. Biosynthesized nanoparticles that combine the unique properties of metals with the bioactivity of C. caesia could be particularly effective in treating wounds, such as cuts and burns [95]. Studies have suggested that gold nanoparticles synthesized from C. caesia rhizomes exhibit benefits in breast cancer treatment. Future research could extend these findings to other cancer types, assessing the potential of various plant parts when combined with nanotechnology for targeted cancer therapy.
C. caesia, has been the focus of several biotechnological studies aimed at improving its propagation, genetic stability, and bioactive compound production. Efficient micropropagation protocols have been developed using rhizome bud explants, supplemented with plant growth regulators like 6-benzyl-amino-purine (BAP) and α-naphthalene acetic acid (NAA), leading to successful shoot proliferation, rooting, and acclimatization [96]. Studies on genetic stability using cytophotometric, random amplified polymorphic DNA (RAPD), and Inter simple sequence repeats (ISSR) markers confirmed that micropropagated plants exhibit genetic uniformity and remain free of somaclonal variations over extended periods in culture [97]. Furthermore, biotechnological advancements such as genetic transformation [98], somatic embryogenesis [99], cryopreservation [100], and marker-assisted breeding [101] are enhancing the conservation, resistance to diseases, and bioactive compound production, particularly curcumin, in Curcuma caesia. These approaches hold significant potential for both large-scale propagation and improving the medicinal qualities of the plant.
The broad applicability of C. caesia also suggests its economic value in generating income through the manufacture of diverse products. Modern medicinal products increasingly focus on skincare, food supplements, and wound-healing materials, among other uses. By utilizing advanced technologies, such as nanotechnology and biotechnology, C. caesia can fulfill the modern market’s growing demands for natural, therapeutic ingredients in health and wellness products [91].
Commercial interest in black turmeric is evident in its inclusion in high-end niche brands and Ayurvedic skincare lines, especially popular in Southeast Asia, Europe, and North America. The global curcumin market was valued at approximately USD 58 million in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 12.7% from 2021 to 2028[102]. Although much of this market value is currently derived from Curcuma longa, the traditional turmeric, demand for the unique bioactive compounds in black turmeric is increasing due to its potency and historical associations in traditional medicine [64].
The herbal cosmetics industry, valued at over USD 80 billion as of 2023, is expanding at a rate of over 5% CAGR[103]. Given the plant’s properties, C. caesia shows considerable promise as a core ingredient in skincare products, offering enhanced product quality and market appeal. With a marked consumer shift towards “clean beauty” and “green cosmetics,” demand for black turmeric is expected to rise as consumers increasingly move away from synthetic ingredients in favor of natural skincare benefits[91]. Black turmeric’s essential oils and curcuminoids make it a valuable component in formulating herbal cosmetics, which are preferred by consumers seeking chemical-free and organic products[38]. Curcuma caesia is especially noted for its natural skin-lightening and anti-acne properties, enhancing its value in the cosmetic industry[104].
In the herbal supplements sector, which includes turmeric-based products, the global market was valued at over USD 6.6 billion in 2020[103, 105, 106]. Black turmeric occupies a niche within this market, with rising sales in the U.S. and Europe, where demand for natural, plant-based health products continues to grow. New formulations, supplements, and extracts of black turmeric in capsule or powder form are opening further market channels, supporting related industries in agriculture, processing, and export[91, 103].
In addition to biomedical applications, C. caesia holds cultural and economic significance, particularly in India and Nepal, where black turmeric is used in rituals and religious festivals, further increasing its demand during these times[107]. This cultural value contributes to its economic impact, supporting a steady local market. Furthermore, with growing interest from international markets in the medicinal and cultural uses of C. caesia, export opportunities provide additional income for cultivators and contribute to foreign exchange earnings. Integrating C. caesia into these markets reflects promising economic and research directions, as biotechnological and nanotechnological advancements continue to drive consumer demand for natural, multifunctional products across healthcare, cosmetics, and nutraceutical sectors[108].
Curcuma caesia also contributes to environmental sustainability and rural economic development. Due to its resilience and ability to grow in varied climates, the black turmeric can be cultivated in challenging areas, promoting soil health and supporting local economies[108, 109]. It can also be incorporated into agroforestry systems or crop rotation practices, fostering biodiversity and providing farmers with supplementary income. Additionally, cultivating, harvesting, and processing black turmeric generates employment opportunities, particularly in rural and tribal regions, thus enhancing livelihoods and contributing to the socioeconomic development of these communities[107, 110].
In light of contemporary healthcare trends, the utilization of bioactive compounds from edible plant species as novel foods and medicinal supplements has gained substantial momentum. The global shift towards healthier dietary choices and well-being, coupled with the cost and adverse effects associated with synthetic medicines, has propelled the popularity of herbal remedies. Notably, plants like C. caesia, boasting a myriad of pharmaceutical properties, are gaining significant appeal. Yet, this ethno-herbal resource remains insufficiently explored, creating a notable gap between traditional use and scientific investigation.
To address this gap, further studies should be conducted to unlock the potential use of not just the rhizomes but also the leaves and stems of C. caesia. Bridging this disparity holds the potential not only to advance medical discoveries but also to have considerable economic implications, potentially bolstering local economies. Increased interest and recognition, particularly among younger generations, could further elevate the plant’s prominence and value, creating a harmonious synergy between traditional wisdom and modern scientific exploration.
References: Click here